We know ice is 0°C and hot water is 80°C, so we could found out efficiency.
Then we started doing a example problem to found COP (coefficient of performance) and Q_h.
We learned how to found effectiveness and did an example problem.
Because we know the reversile process is constant, so we could found the final temperature.
Then we found out the efficiency of the Carnot cycle and the efficiency of the reversile process.
There is another example problem for us to solve Q_c and Q_h. After we known Q_c and Q_h, we need to found how long could 4.2kg of water freeze to ice.
Last, we did a bubble demo. Bubbles will fell down if the bubbles make by air. However, bubbles will go up if the bubbles make by gas.
Today, we did two experiments and many example problems. For the Stirling cycle experiment, the fan will rotate if we put ice on top and hot water at bottom. Also, it will rotate opposite direction if we put hot water on top and ice at bottom. We could use T_h/(T_h-T_c) to find COP. For the bubble experiment, we understood that bubble will fells down if it makes by air and goes up if it makes by gas.




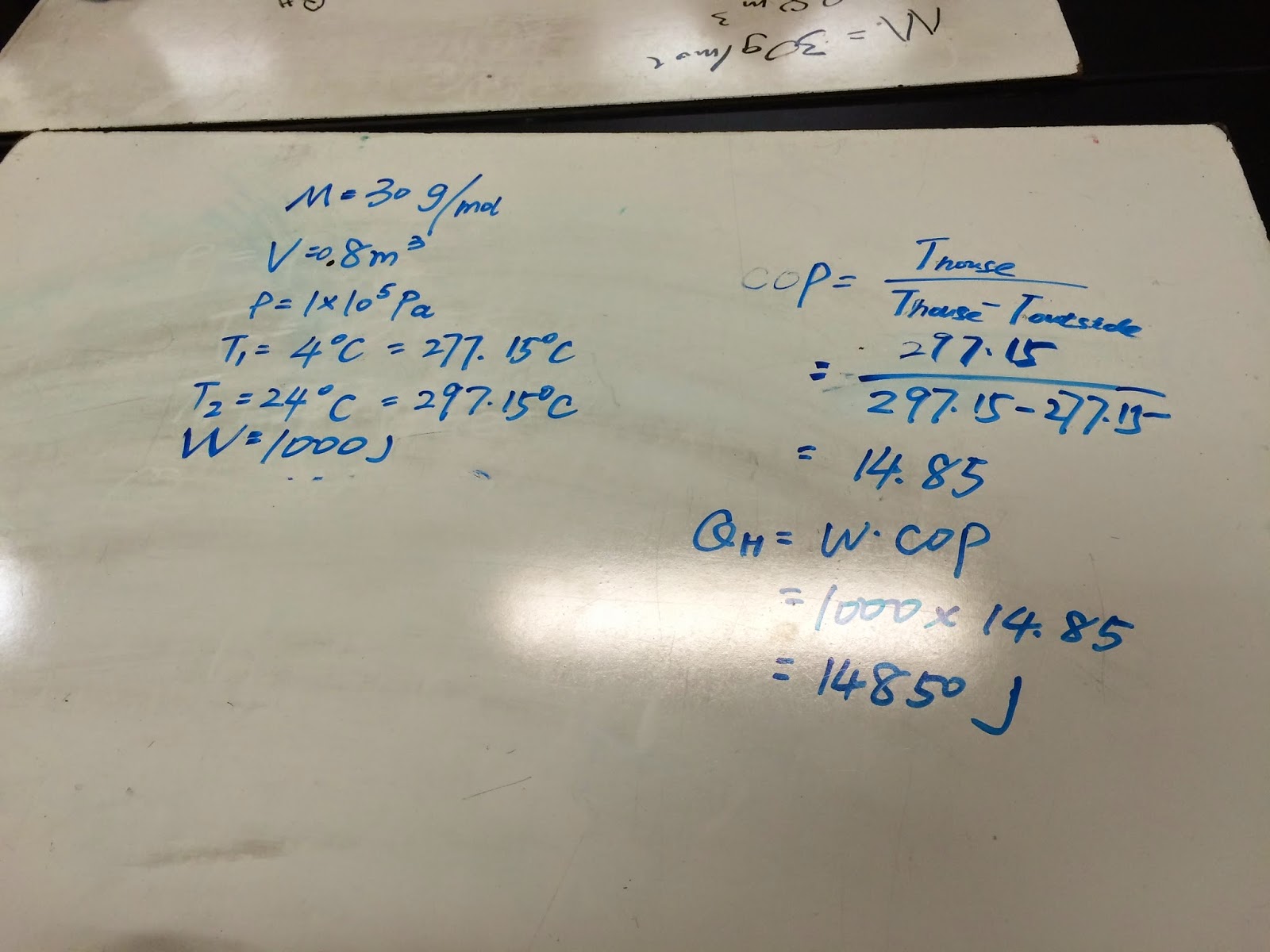








No comments:
Post a Comment