The first thing we did in class is to find the linear equation of Fahrenheit and Celsius.We plug in numbers into the general linear equation y=mx+b. Then the final equation we found is F=(9/5)(C+32).
We were trying to found out the temperature of the room in Kelvin. The temperature we found is 295.15K. Each group had different number. However, the average of temperatures is 295.33K. Then we used standard deviation equation to found the uncertainty. The uncertainty we found is 1.30.
Professor Mason mixed a cup of hot water and a cup of cold water and wanted us to found final temperature. We knew the masses and temperatures of water, so we set Qsystem = -Qsurrounding. Also Q=mc(Tf-Ti). The final temperature we found is 47.8C.
A 50g aluminum can contained 150g cold water and surrounded by 137g hot water. We were trying to found the specific heat of the aluminum can.
This picture shows heat flow=kA((Tf-Ti)/L).
There is an example question, we needed to found the final temperature of aluminum by using dQ/dt=(A(Tf-Ti))/R.
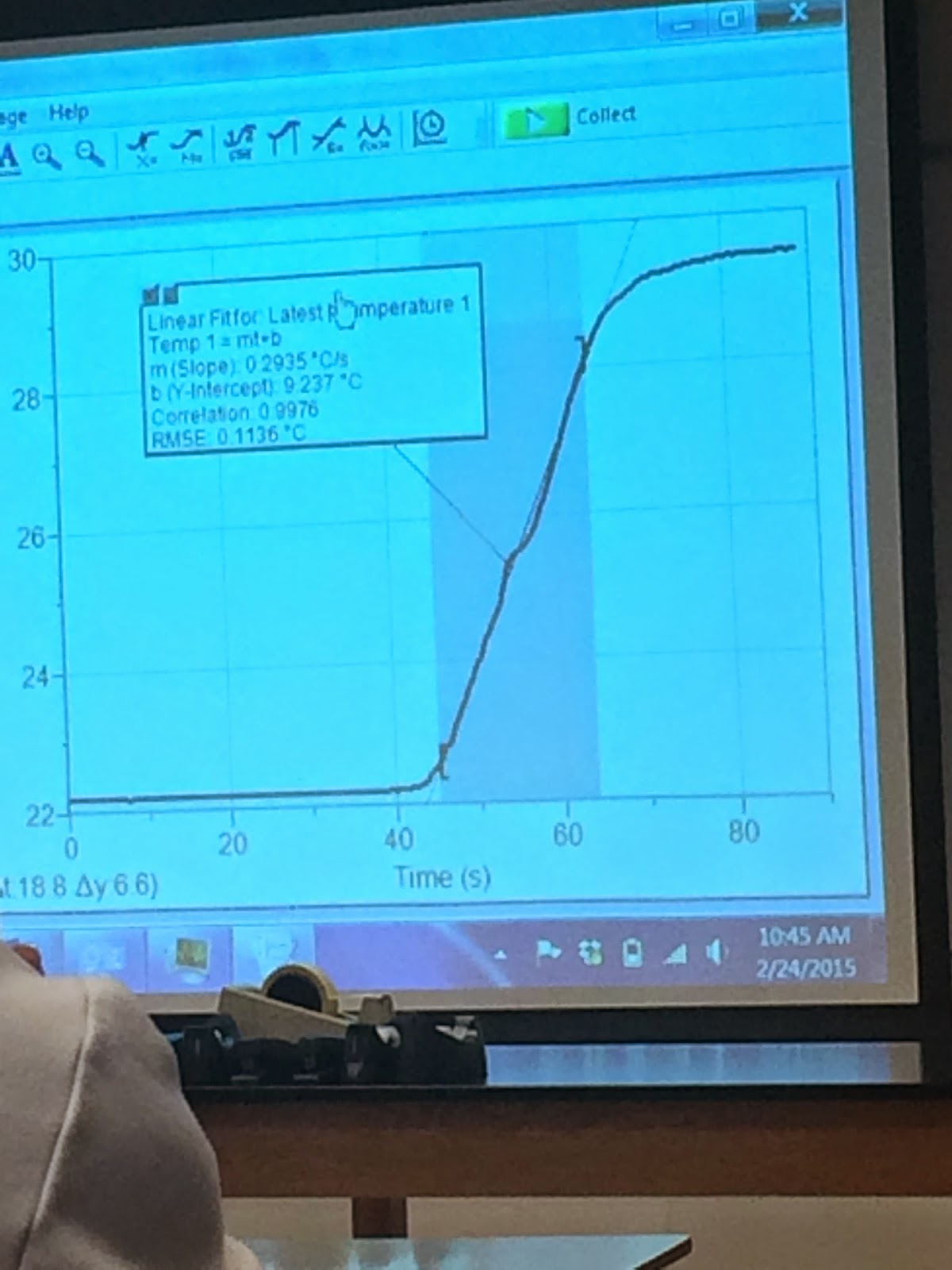
We were using a temperature sensor to collect temperature changing data of a heater.
Then we figured out Q=(995.3)x-2.1133E+0.04 by using the graph that above and general linear equation y=mx+b.
Conclusion:
Today, we learned units conversion of temperature, concepts of thermal conductivity and heat transfer. This lab is very helpful, it makes us more easier to understand all these equations and how it works like dQ/dt=kA((Tf-Ti)/L). Also, all these tools and senors are really helpful for collecting data, like using temperature senor to collect heater temperature changing data.